

 I bought this unusual-looking half dollar in an eBay auction toward the end of 2003. At a reported weight of 7.5 grams, it was 4 grams lighter than a normal half dollar of this era should be. NGC had labeled it as having been struck on “10c thickness stock”. This ambiguous label could mean it was struck on copper-nickel clad dime stock or, perhaps, 40% silver half dollar stock rolled to dime thickness. The listed weight of 7.5 grams was not consistent with either interpretation. A half dollar stuck on a planchet punched out of copper-nickel clad dime stock should weigh around 6.67 grams. A half dollar struck on a planchet punched out of 40% silver half dollar stock rolled to dime thickness should weigh approximately 7.3 grams. So it was too heavy for both.
I bought this unusual-looking half dollar in an eBay auction toward the end of 2003. At a reported weight of 7.5 grams, it was 4 grams lighter than a normal half dollar of this era should be. NGC had labeled it as having been struck on “10c thickness stock”. This ambiguous label could mean it was struck on copper-nickel clad dime stock or, perhaps, 40% silver half dollar stock rolled to dime thickness. The listed weight of 7.5 grams was not consistent with either interpretation. A half dollar stuck on a planchet punched out of copper-nickel clad dime stock should weigh around 6.67 grams. A half dollar struck on a planchet punched out of 40% silver half dollar stock rolled to dime thickness should weigh approximately 7.3 grams. So it was too heavy for both.
 A weight of 7.5 grams is consistent with a half dollar struck on a 21% silver core. Each clad layer weighs approximately 2 grams, so a loss of both would yield the reported weight. One such half dollar is actually known. However, an exposed core displays a gray, streaky appearance. This coin showed a uniformly bright, silvery appearance. So I didn’t think that was likely.
A weight of 7.5 grams is consistent with a half dollar struck on a 21% silver core. Each clad layer weighs approximately 2 grams, so a loss of both would yield the reported weight. One such half dollar is actually known. However, an exposed core displays a gray, streaky appearance. This coin showed a uniformly bright, silvery appearance. So I didn’t think that was likely.
 The weight could be might also be consistent with a “split core” error of just the right thickness. The unstruck perimeter of the reverse does show fine striations that are not present on the obverse.
The weight could be might also be consistent with a “split core” error of just the right thickness. The unstruck perimeter of the reverse does show fine striations that are not present on the obverse.
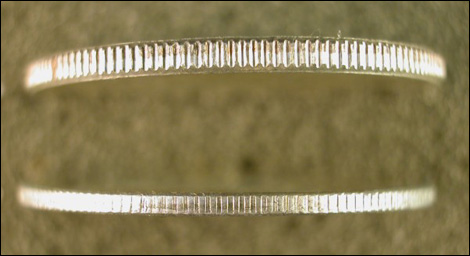
However, the striations appear too fine to represent a fracture plane, and, anyway, the reverse does not show the gray, streaky color of an exposed core.
 Interestingly, a weight of 7.5 grams is consistent with a half dollar punched out of 90% silver dime stock. A transitional wrong stock error would be quite a find, so I decided to buy the coin and crack it out.
Interestingly, a weight of 7.5 grams is consistent with a half dollar punched out of 90% silver dime stock. A transitional wrong stock error would be quite a find, so I decided to buy the coin and crack it out.
 The first thing I did after liberating the coin was to weigh it on my analytical balance. It came in at 7.46 grams. So far, so good.
The first thing I did after liberating the coin was to weigh it on my analytical balance. It came in at 7.46 grams. So far, so good.
 Edge-on, the coin is almost as thin as a dime Apart from a few short, dark streaks, it betrayed no sign of a 79% copper core, which in normal half dollars usually shows up as a continuous dark gray band.
Edge-on, the coin is almost as thin as a dime Apart from a few short, dark streaks, it betrayed no sign of a 79% copper core, which in normal half dollars usually shows up as a continuous dark gray band.

The edge shows weak reeding all around, which demonstrates that the coin’s diameter matches that of a normal half dollar. The strike is weak, especially at the perimeter, which is what you’d expect of a coin this thin.

 Although I knew that not all 40% silver half dollar show obvious evidence of a core, I was still optimistic I was dealing with a solid 90% silver alloy. That was my expectation when I subjected the coin to density test known as a specific gravity (SG) test. That test confounded my expectations as it came out to a very low 9.1. A 90% silver coin should have a specific gravity of 10.34 (Margolis and Weinberg, 2000). I ran the test twice to make sure that my results were accurate.
Although I knew that not all 40% silver half dollar show obvious evidence of a core, I was still optimistic I was dealing with a solid 90% silver alloy. That was my expectation when I subjected the coin to density test known as a specific gravity (SG) test. That test confounded my expectations as it came out to a very low 9.1. A 90% silver coin should have a specific gravity of 10.34 (Margolis and Weinberg, 2000). I ran the test twice to make sure that my results were accurate.
 A specific gravity result of 9.1 is very low. The listed SG of a normal 40% silver half dollar is 9.53. The listed SG of a copper-nickel clad half dollar is 8.92. So my half dollar was closer to copper-nickel than the normal silver-and-copper composition.
A specific gravity result of 9.1 is very low. The listed SG of a normal 40% silver half dollar is 9.53. The listed SG of a copper-nickel clad half dollar is 8.92. So my half dollar was closer to copper-nickel than the normal silver-and-copper composition.
 I now needed to verify that the observed mean SG in a sample of 40% silver halves matched the listed mean SG of 9.53 reported in The Error Coin Encyclopedia. I also wanted to compare it to the theoretical mean density of a disc composed of 40% silver and 60% copper. The specific gravity of elemental silver is 10.5 while that of elemental copper is 8.96 (Handbook of Chemistry and Physics). A weighted average of the two densities yields a theoretical value of 9.58 for a 40% silver half. In addition to calculating a mean, I also wanted to establish a range of variation. To that end I performed specific gravity tests on a sample of ten 40% silver halves from the years 1965 to 1969. The mean SG value was 9.45. The observed range was 9.37 to 9.54. The standard deviation was 0.063.
I now needed to verify that the observed mean SG in a sample of 40% silver halves matched the listed mean SG of 9.53 reported in The Error Coin Encyclopedia. I also wanted to compare it to the theoretical mean density of a disc composed of 40% silver and 60% copper. The specific gravity of elemental silver is 10.5 while that of elemental copper is 8.96 (Handbook of Chemistry and Physics). A weighted average of the two densities yields a theoretical value of 9.58 for a 40% silver half. In addition to calculating a mean, I also wanted to establish a range of variation. To that end I performed specific gravity tests on a sample of ten 40% silver halves from the years 1965 to 1969. The mean SG value was 9.45. The observed range was 9.37 to 9.54. The standard deviation was 0.063.
 The anomalous 1965 half dollar was over 5 standard deviation units from the mean. Anything over 2 standard deviation units is considered statistically significant. If this half dollar was composed of silver and copper, it would have to be in a ratio of 10% silver to 90% copper!
The anomalous 1965 half dollar was over 5 standard deviation units from the mean. Anything over 2 standard deviation units is considered statistically significant. If this half dollar was composed of silver and copper, it would have to be in a ratio of 10% silver to 90% copper!
 At this point all I knew was that the half dollar was abnormally thin and light, with an abnormally low density. I couldn’t tell if it was a solid alloy, a clad composition, or something more exotic, like a silver-plated composition. So I sent it off to metallurgist and numismatist Chris Pilliod for a high-energy, semi-quantitative x-ray analysis (SEM/X-ray). The results of that analysis were equally puzzling.
At this point all I knew was that the half dollar was abnormally thin and light, with an abnormally low density. I couldn’t tell if it was a solid alloy, a clad composition, or something more exotic, like a silver-plated composition. So I sent it off to metallurgist and numismatist Chris Pilliod for a high-energy, semi-quantitative x-ray analysis (SEM/X-ray). The results of that analysis were equally puzzling.
 Three analyses were performed on both the obverse and reverse face, with each test at a different voltage. Higher voltage results in increased depth of penetration. I only received a printout of the report on the obverse face but was assured that the reverse face produced identical readings.
Three analyses were performed on both the obverse and reverse face, with each test at a different voltage. Higher voltage results in increased depth of penetration. I only received a printout of the report on the obverse face but was assured that the reverse face produced identical readings.
 At the lowest voltage – 15 keV – the obverse showed a composition of 91% silver and 9% copper. At a voltage of 20 keV, it showed a composition of 71% silver and 29% copper. At a voltage of 30 keV, it showed a composition of 57% silver and 43% copper. These three tests revealed that the coin was not a solid alloy but, instead, consisted of silver or a silver/copper alloy overlying a core of copper or copper/silver alloy.
At the lowest voltage – 15 keV – the obverse showed a composition of 91% silver and 9% copper. At a voltage of 20 keV, it showed a composition of 71% silver and 29% copper. At a voltage of 30 keV, it showed a composition of 57% silver and 43% copper. These three tests revealed that the coin was not a solid alloy but, instead, consisted of silver or a silver/copper alloy overlying a core of copper or copper/silver alloy.
 The shallowest penetration would be the most accurate indicator of surface metal composition. According to Chris, at 15 keV, only the top few layers of atoms are sampled. 91% silver is considerably higher than the 80% silver that a standard 40% silver half dollar is supposed to have. There are four possible interpretations of this anomalous value.
The shallowest penetration would be the most accurate indicator of surface metal composition. According to Chris, at 15 keV, only the top few layers of atoms are sampled. 91% silver is considerably higher than the 80% silver that a standard 40% silver half dollar is supposed to have. There are four possible interpretations of this anomalous value.
1. Experimental error. Since results obtained for the obverse and reverse matched, this doesn’t seem to be likely.
2. Depletion of copper from the surface. This would seem unlikely in a coin that, when first taken from its slab, was bright and untarnished.
3. An accurate reading of a clad layer abnormally rich in silver.
4. A thin clad layer of pure silver or thin layer of pure silver plating, with the 9% copper signal leaking through from the core
 inspected the cut, the underlying metal gleamed with a bright copper color. I figured an ordinary 40% silver half would show the gray color that the edge of an unmarked coin usually shows. This ultimately proved to be an unreliable diagnostic, because when I cut into the edge of a normal half dollar with a scalpel, the underlying metal also had a bright copper color. The gray color must be due to copper depletion at the surface or the effects of the chemical rinse bath that planchets are immersed in prior to the strike.
inspected the cut, the underlying metal gleamed with a bright copper color. I figured an ordinary 40% silver half would show the gray color that the edge of an unmarked coin usually shows. This ultimately proved to be an unreliable diagnostic, because when I cut into the edge of a normal half dollar with a scalpel, the underlying metal also had a bright copper color. The gray color must be due to copper depletion at the surface or the effects of the chemical rinse bath that planchets are immersed in prior to the strike.
 Only one test of the edge was undertaken and it showed a ratio of 95% copper to 5% silver. The core of a normal 40% silver half dollar should have a composition of 79% copper and 21% silver.
Only one test of the edge was undertaken and it showed a ratio of 95% copper to 5% silver. The core of a normal 40% silver half dollar should have a composition of 79% copper and 21% silver.
 Chris warned me that readings taken from the edge are notoriously unreliable. Be that as it may, we can assign three possible interpretations to this result:
Chris warned me that readings taken from the edge are notoriously unreliable. Be that as it may, we can assign three possible interpretations to this result:
1. Experimental error. Without additional tests, there’s no way to know how reproducible these results are.
2. An accurate reading of a 95% copper core.
3. The core is actually pure copper, with the residual 5% silver coming from the metal surrounding the cut in the edge.
After all this testing and analysis, what conclusions can I draw?
 Chris Pilliod believes it’s simply a half dollar struck on rolled-thin 40% silver clad stock. However, in my view, the specific gravity test, SEM/X-ray results, and the striated reverse perimeter are not consistent with this interpretation. Again, the specific gravity results would indicate an aggregate composition of 10% silver and 90% copper.
Chris Pilliod believes it’s simply a half dollar struck on rolled-thin 40% silver clad stock. However, in my view, the specific gravity test, SEM/X-ray results, and the striated reverse perimeter are not consistent with this interpretation. Again, the specific gravity results would indicate an aggregate composition of 10% silver and 90% copper.
 It could possibly be a half dollar struck on rolled-thin 40% silver clad stock with abnormal proportions of silver and copper in both clad layers and the core. However, given the high percentage of copper that would have to be present in the core, I would have expected the core to show up on the edge as a continuous coppery or dark band. The core is not evident on the edge. Apart from a few thin, short, irregular, dark streaks, the edge has the same bright silvery appearance as the two faces. The roughened, striated reverse perimeter is also not consistent with a simple rolled-thin error.
It could possibly be a half dollar struck on rolled-thin 40% silver clad stock with abnormal proportions of silver and copper in both clad layers and the core. However, given the high percentage of copper that would have to be present in the core, I would have expected the core to show up on the edge as a continuous coppery or dark band. The core is not evident on the edge. Apart from a few thin, short, irregular, dark streaks, the edge has the same bright silvery appearance as the two faces. The roughened, striated reverse perimeter is also not consistent with a simple rolled-thin error.
 It could be a planchet composed of pure silver plating over a pure copper core. This would be consistent with the low specific gravity, the SEM/X-ray results, the striated reverse perimeter (not fully obscured by the thin plating), and the failure of the core to show up on the edge.
It could be a planchet composed of pure silver plating over a pure copper core. This would be consistent with the low specific gravity, the SEM/X-ray results, the striated reverse perimeter (not fully obscured by the thin plating), and the failure of the core to show up on the edge.
 If it is a silver-plated, pure copper planchet, perhaps it was not intended to be struck as a half dollar at all. Silver-over-copper is a composition I’ve seen in tokens and in counterfeit coins. Perhaps a half dollar-sized planchet intended for a token or medal ended up in a half dollar hopper.
If it is a silver-plated, pure copper planchet, perhaps it was not intended to be struck as a half dollar at all. Silver-over-copper is a composition I’ve seen in tokens and in counterfeit coins. Perhaps a half dollar-sized planchet intended for a token or medal ended up in a half dollar hopper.
 Finally, it could be an experimental coin. During the run-up to the transition from silver to clad coins, numerous experimental compositions were tested (Bowers, 2003). But why would anyone make an experimental half dollar planchet that was so darned thin?
Finally, it could be an experimental coin. During the run-up to the transition from silver to clad coins, numerous experimental compositions were tested (Bowers, 2003). But why would anyone make an experimental half dollar planchet that was so darned thin?
 I suppose this coin is destined to remain a conundrum (or should I say “coinundrum”).
I suppose this coin is destined to remain a conundrum (or should I say “coinundrum”).


References
Bowers, Q. David, editor (2003) United States Pattern Coins, Experimental and Trial Pieces by J. Hewitt Judd, p. 289
Margolis, Arnold and Weinberg, Fred. (2000) The Error Coin Encyclopedia, 4th edition, pp. 443 – 444
Weast, Robert C. (1970) Handbook of Chemistry and Physics, 51st edition. Cleveland: The Chemical Rubber Company

